Boiling Point of Hcl and F2
F2 Cl2 Br2. F2 has van der Waals forces whilst HCl has permanent dipoledipole attractions.

Co 2 Ar H 2 Ar Geothermometer Grid R H 2 8 With Gas Data From Download Scientific Diagram
Gases generated in a chemical reaction are sometimes collected by the displacement of water as shown above.
. Permanent dipoledipole attractions are much stronger than induced dipoles. What is the boiling point of HF and HCL. Hydrogen chloride boils at 85 C and fluorine boils at 188 CExplain why there is a difference in the boiling points of HCl and F2.
Which fact adequately explains why HCl has a higher boiling point than F2. Does HF have a high boiling point. They can be easily broken into its primary structure as the atoms are close by to share the electrons.
If we talk about the HF it has strong hydrogen bonding thus has the highest boiling point. F2 HCI LiF HF O d. Why is the boiling point of HCl lowest while that of HF highest.
Chemistry questions and answers. CH4 C2H6 C3H8. The boiling point of hydrogen is -253 degrees Celsius and the boiling point of fluorine is -188 degrees Celsius.
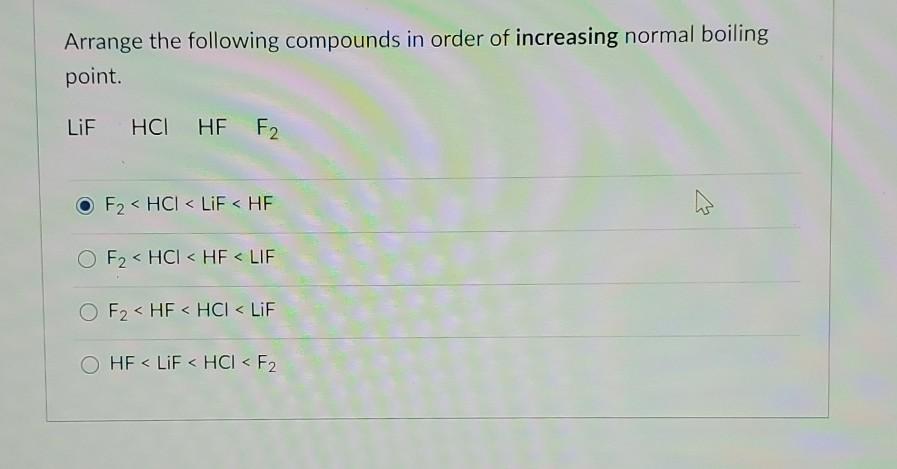
Also consider that water molecules are made up of two hydrogen atoms and one oxygen atom thus having two bonds rather than one. The boiling point of hydrogen bromide HBr -6638oC and the boiling point of hydrogen chloride HCl is -851oC. Arrange LiF HCl HF and F2 in order of increasing normal boiling point.
Bigger molecules will have stronger London dispersion forces. HF is hydrogen bonded thus has highest boiling point and it is liquid at or below 19 oC. HF LiF HCI F2.
Therefore HF exists in the liquid state and HI HBr and HCl exists in the gaseous state. None of these have hydrogen bonding. OF is a polar molecule HCI is not O HCl is a polar molecule F2 is not OF is heavier than HCI OHCl is heavier than F.
Why does HF have a higher boiling point than F2. N 2H ClH 2ON aCl. F2 is very small hence strong forces of attraction.
F2 HCI HF LiF O c. The remaining hydrogen halides are gaseous and their boiling points depend on the van der waals forces. The boiling point of hydrogen flouride HF is the highest at 195oC.
F2. Go through the list above. Which fact adequately explains why HCl has a higher boiling point than F2.
HCl HF F2 E. HF has a normal boiling point of 195 C whereas that of HCl is 850 C. More enegery is required to break the stronger permanent dipoledipole attractions and therefore HCL has.
1None of these have hydrogen bonding. Which of the following gases can be quantitatively. Using your chemical intuition assign the highest and lowest boiling compounds and provide a brief explanation.
Br2 F2 I2 Cl2 Answer Higher boiling points will correspond to stronger intermolecular forces. The order of increasing boiling points is going to follow increasing inter molecular force strength. So I2 has the strongest forces and F2 will have the weakest.
Arrange each of the following sets of compounds in order of increasing boiling point temperature. List the following molecules in order of increasing boiling point. Arrange each of the following sets of compounds in order of increasing boiling point temperature.
-85 o C even though HF has a lower molecular weight. The boiling point of hydrogen is -253 degrees Celsius and the boiling point of fluorine is -188 degrees Celsius. Select the correct answer below.
None of these have dipoles. Molecules of hydrogen chloride HCl and molecules of fluorine F2 contain the same number of electrons. F2 HF HCI LiF b.
F2 has van der Waals forces whilst HCl has permanent dipoledipole attractions. I would expect HCl to have a higher boiling point than HF. Larger the size or molecular mass greater are the van der Waals forces hence higher is the boiling point.
Chemistry questions and answers. 2None of these have dipoles. The boiling points of HFHClHBr and HI follow the order HFHIHBrHCl.
Arrange LiF HCl HF and F2 in order of increasing normal boiling point. Data sheet and Periodic Table Select one. F2 Cl2 Br2 I2.
You look at the first three substances Hcl HBR and H. A The intermolecular bonding for HF is van der Waals whereas for HCL the intermolecular bonding is hydrogen. Why does HF have a higher boiling point.
HF has a higher boiling point than F2 because more effort is needed to make H and F atoms stop being close to each other electrostatic attraction causes close proximity13 Sept 2020. Which of the following lists the substances F₂ HCL and HF in order of increasing boiling point A. Cl2 Chlorine molecule has a boiling point closest to that of argon.
If we talk about the HF it has strong hydrogen bonding thus has the highest boiling point. The best metric for this INTERMOLECULAR force is the boiling point. Both HCl and HF have higher boiling points due to hydrogen bonding and should fall between the two extremes.
We can conclude the order of boiling point followed is HF HI HBr HCl. 3Bigger molecules will have stronger London dispersion forces. The molecules associate through weak van der waals forces of attraction hence low boiling point.
Correspondingly I2 will have the highest boiling point and F2 will have the lowest boiling point. HCl Boiling point 851 C 1879 K F2 Boiling point 8503 K 18812 C 30662 F NaF Boiling point 1695 C HF Boiling point 195 C 293 K 67 F Boiling Point Of Ar. HF shows H-bonding possess a higher boiling point or less volatile nature.
0 g of ethanol C2H5OH are vaporized at. Because there is greater polarity in the HF bond the opportunity for hydrogen bonding in the bulk solvent is greater. HCl has the least boiling point due to the small dispersion intermolecular forces.
HF F2. HCl F2 HF D. F2 HCl HF.
HCl has strong hydrogen bonds. SiH4 HCl H2O. Explain why hydrogen fluoride HF has a higher boiling temperature than hydrogen chloride HCL 194 o C vs.
The lowest should be F2 since there is no hydrogen bonding involved. Order of increasing boiling point. Due to a lesser number of electrons HCl has weaker Van der Waals forces London dispersion forces in this case than HBr resulting in a lower boiling point.
Go through the list above. The presence of Hydrogen bonding is negligible in these halides. Chemistry QA Library Arrange LiF HCI HF and F2 in order of increasing normal boiling point.
HF HCl F2 B. The stronger the inter molecular force the higher the boiling point the lower the vapor pressure but the higher the boiling point and the higher the melting point. This is because Chlorine is made up of discrete and simple molecules making up a simple molecular structure.
Halogens Atomic radius Melting point Boiling point Fluorine F2 38 gmol 72 pm 53 K 85 K chlorine Cl2 71 gmol 99 pm 172 K 238 K bromine Br2 160 gmol 114 pm 266 K 332 K iodine I 2254 gmol 133 pm 387 K 457 K astatin At2 420 gmol PM 575 K 610 k The increase in fusion and boiling points with the increasing atomic molecular dimensions.
Solved Arrange The Following Compounds In Order Of Chegg Com

Arrange Each Group Of Compounds In Order Of Increasing Boiling Point Explain F2 Cl2 Br2

Invitro Drug Release Profile Of Formulation F1 F2 F3 Download Scientific Diagram

Higuchi Model For Formulation F F1 F2 And F3 Download Scientific Diagram

Higuchi Model For Formulation F F1 F2 And F3 Download Scientific Diagram

Pharmaceuticals Free Full Text Design Green Synthesis And Tailoring Of Vitamin E Tpgs Augmented Niosomal Nano Carrier Of Pyrazolopyrimidines As Potential Anti Liver And Breast Cancer Agents With Accentuated Oral Bioavailability Html
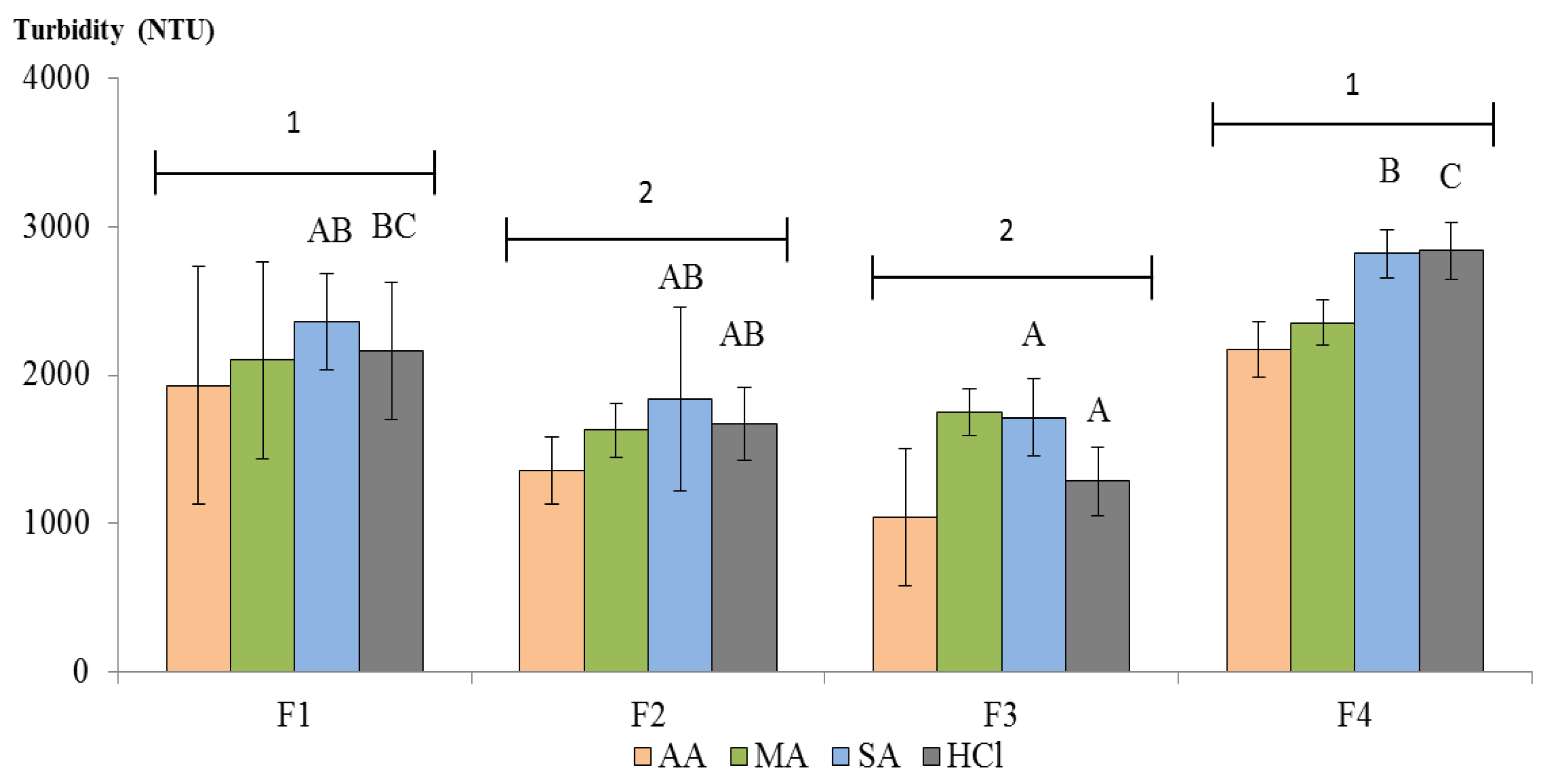
Applied Sciences Free Full Text Shellfish Chitosan Potential In Wine Clarification Html
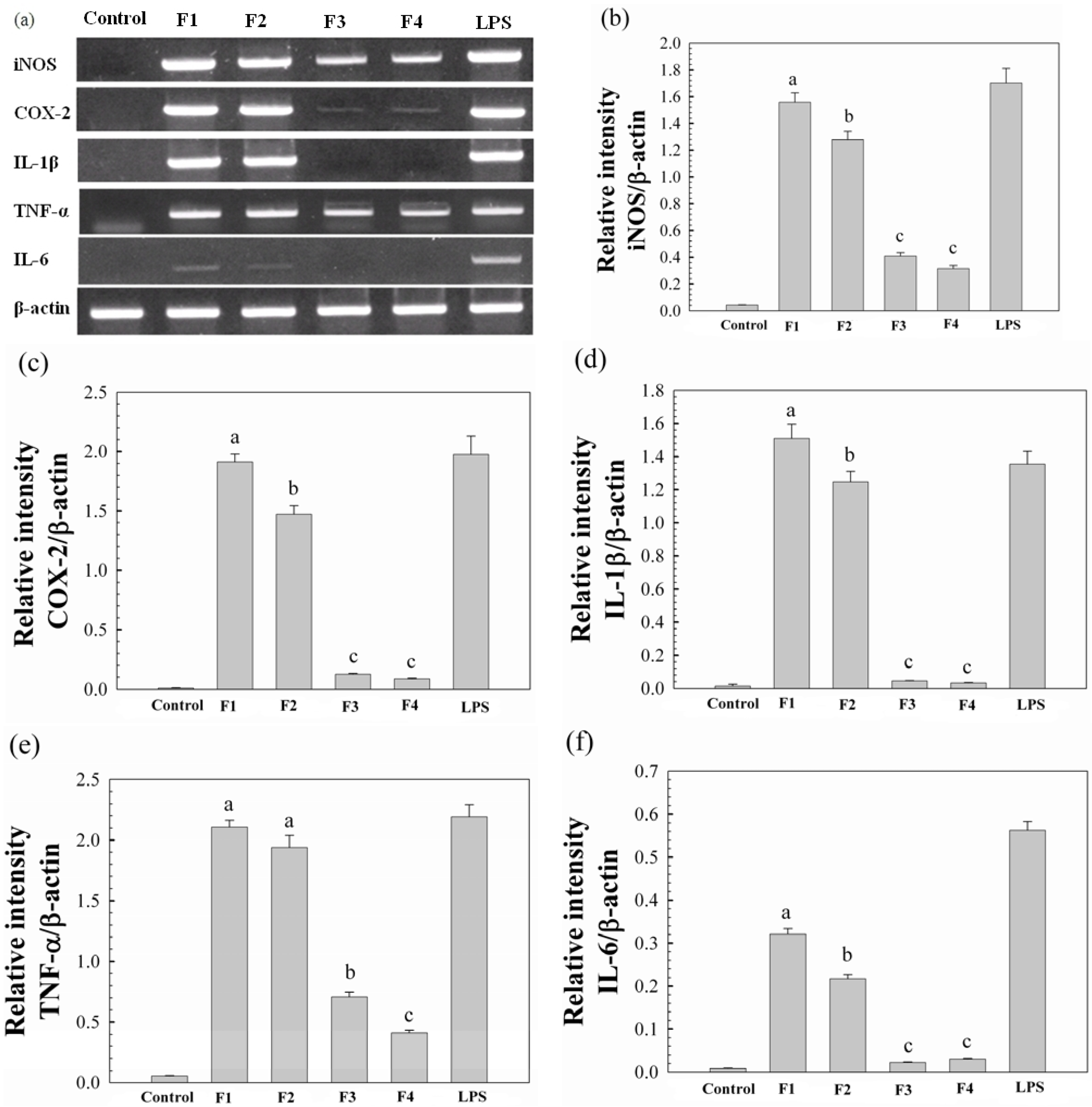
Molecules Free Full Text Molecular Properties Of Water Unextractable Proteoglycans From Hypsizygus Marmoreus And Their In Vitro Immunomodulatory Activities Html
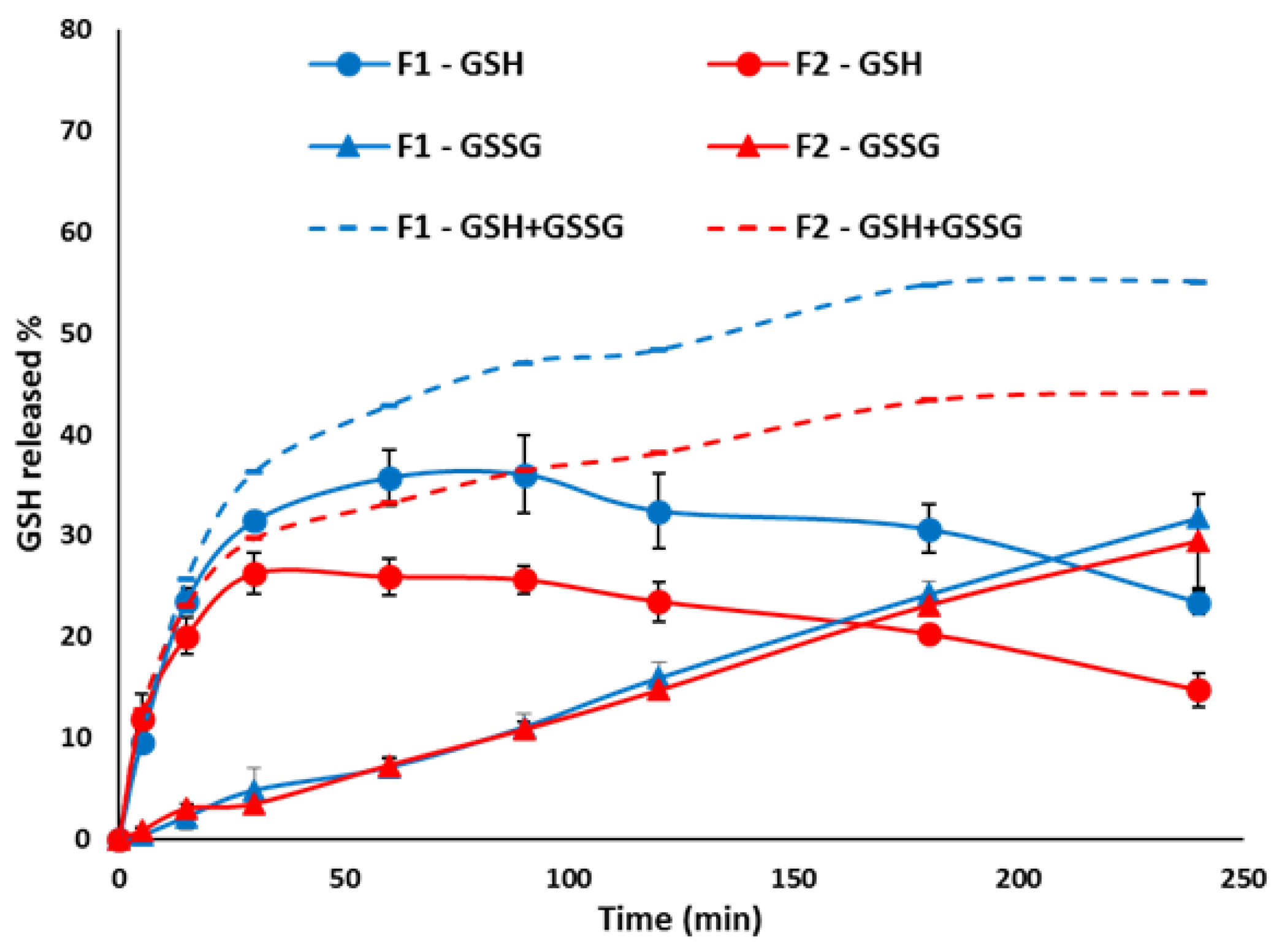
Pharmaceutics Free Full Text Glutathione Loaded Solid Lipid Microparticles As Innovative Delivery System For Oral Antioxidant Therapy Html

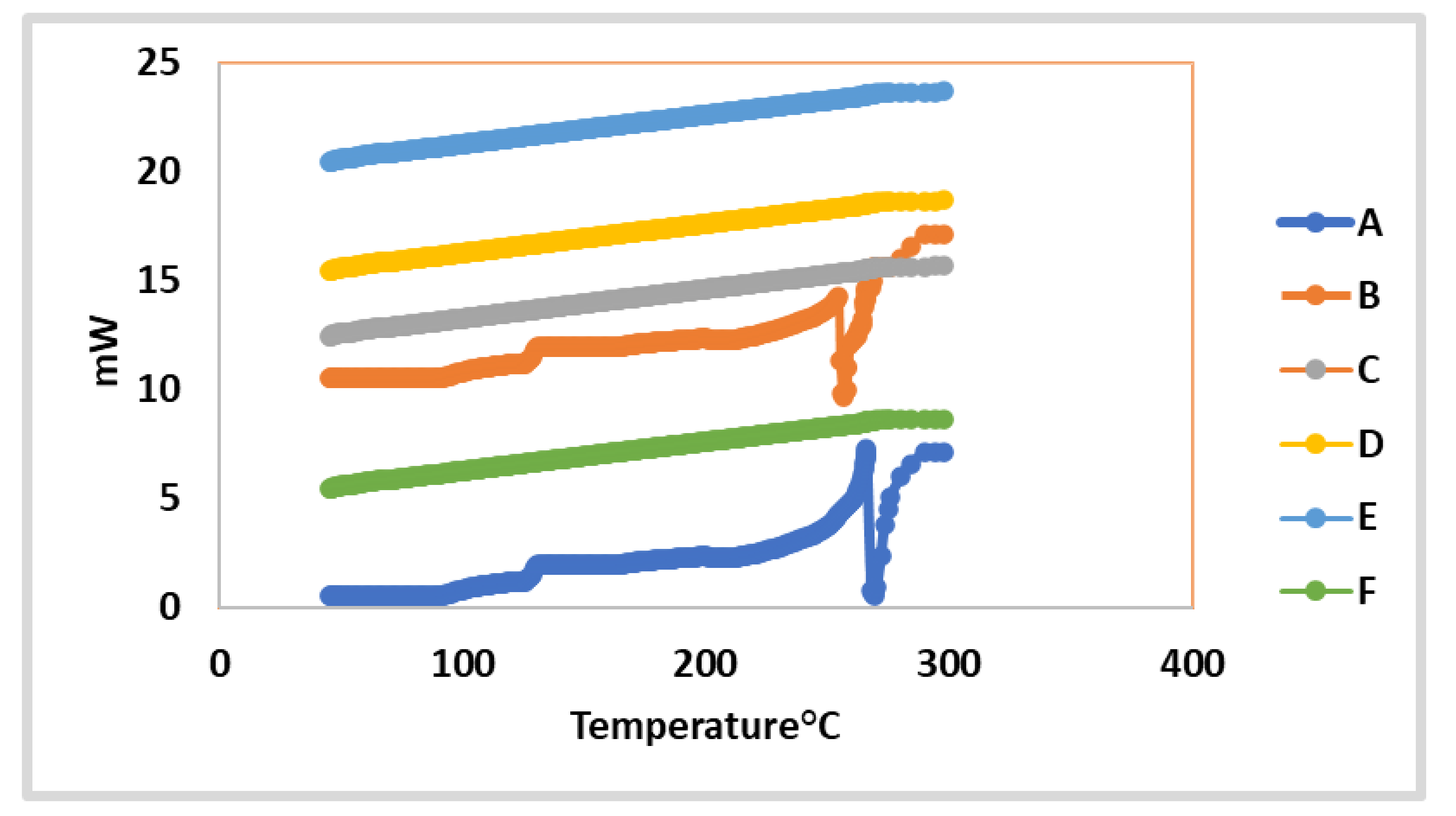
Dsc Thermograms Of F1 Untreated Maltodextrin F2 Treated Maltodextrin Download Scientific Diagram
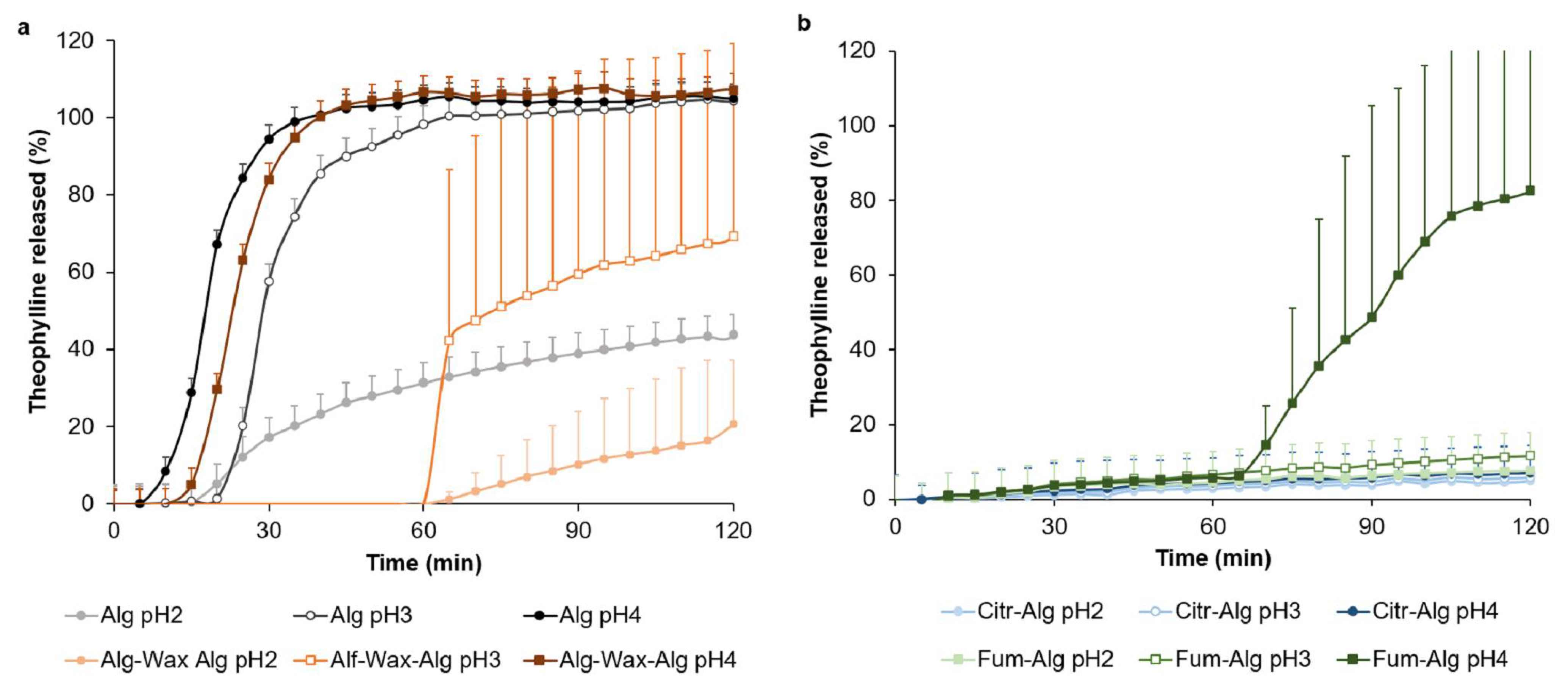
Pharmaceutics Free Full Text A Novel Multilayer Natural Coating For Fed State Gastric Protection Html

Melting Point Trend For Group 17 Halogens F2 Cl2 Br2 I2 Youtube

Invitro Drug Release Profile Of Formulation F1 F2 F3 Download Scientific Diagram
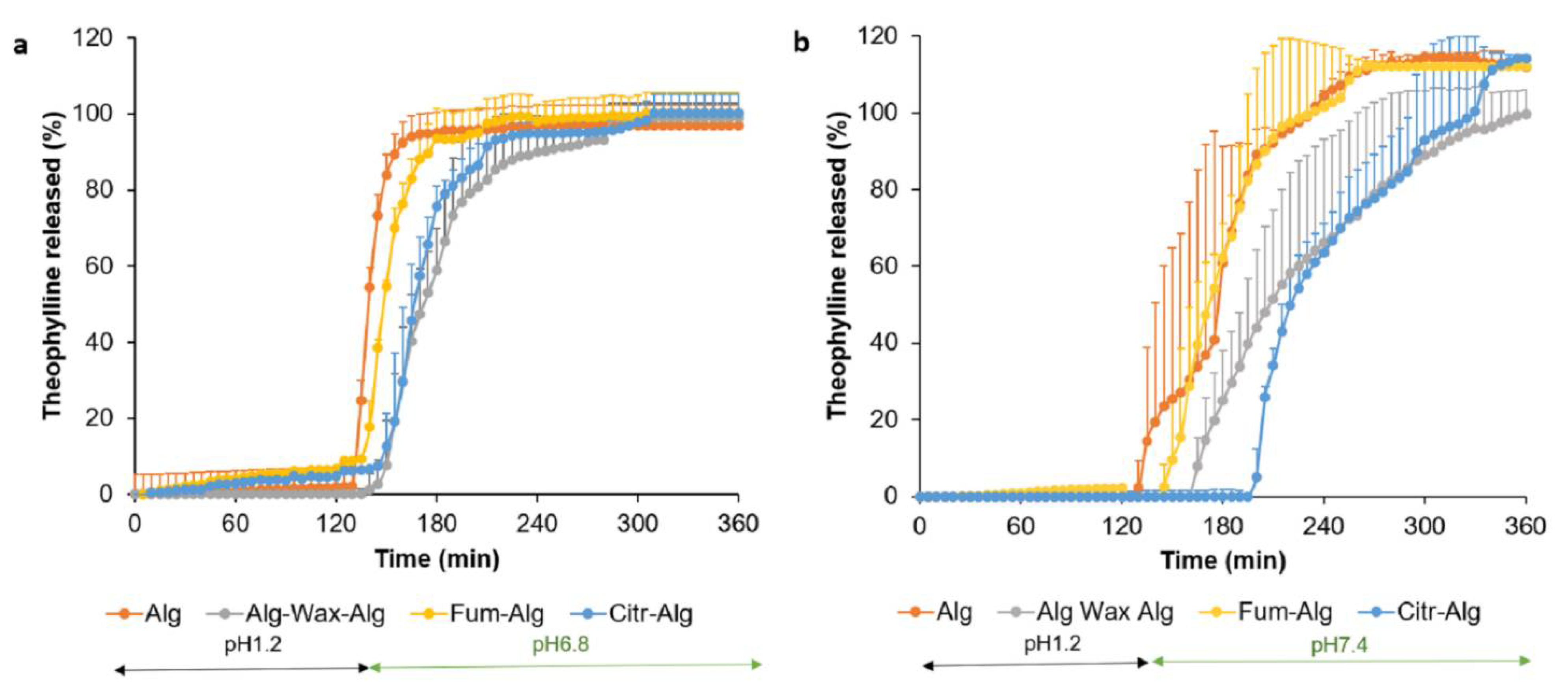
Pharmaceutics Free Full Text A Novel Multilayer Natural Coating For Fed State Gastric Protection Html

Comparative Dissolution Profile Of Amlodipine Besylate Tablets Download Scientific Diagram

Why Is The Boiling Point Of F2 Fluorine So Low Youtube

Temperature Profiles Determined For F1 And F2 Furnace Configurations Download Scientific Diagram


Comments
Post a Comment